
In the life sciences industry, quality management is not only directly related to patient safety and treatment effectiveness, but also affects the sustainable development and brand reputation of enterprises. Faced with increasingly strict regulatory requirements, enterprises need to start from product research and development to market launch, production, and even supply chain, and implement rigorous quality management in every detail.
According to the FDA report, the pharmaceutical supply chain continues to face challenges such as product recalls, drug shortages, and supply disruptions due to substandard production quality. In 2022, the FDA issued over 230 temperature related recalls and 51 production recalls related to the use of contaminated excipients that were recalled. Solving and preventing these problems is not only time-consuming and costly, but also can cause significant damage to the company's reputation.
The environment of the biopharmaceutical industry is becoming increasingly complex, facing multiple challenges such as dynamic regulatory requirements, shortage of skilled labor, surge in demand for personalized medicine, and intensified competition. Adopting digital solutions, such as structured document management systems, will greatly help enterprises ensure the accuracy of information throughout the entire product development lifecycle.
BIOVIA launches a new generation of comprehensive quality management solutions aimed at helping life science enterprises cope with increasingly complex and changing regulatory requirements, ensure regulatory compliance, improve operational efficiency, and comprehensively build a strong defense line against potential risks.
Unleash the full potential of quality management
Integrated platform:
Based on the 3DEXPERIENCE platform, fully integrate quality management processes, simplify document processing, and ensure compliance at all levels of operation.
Efficient data-driven automation:
Centralize and securely manage data from multiple sources, automate content generation, and reduce manual tasks by up to 70%.
AI driven insights promote proactive management:
Helping enterprises predict and prevent potential problems, combined with virtual twin technology, to achieve a transition from passive response to proactive management, ensuring the achievement of higher quality standards.
Exclusive advantage
Chemistry, Production, and Control (CMC) is the core of submitting drug declarations to the FDA or other regulatory agencies, and is also the key to successful registration and declaration. The CMC file is very complex, and its preparation work is also time-consuming and laborious. BIOVIA has pioneered a new method for compiling CMC documents. Transitioning from traditional static documents to automated archive creation centered around data. In BIOVIA Structured Document Writing, documents are presented in a combination of content and data.
The data, results, and conclusions that were originally scattered across various systems can be seamlessly integrated into a unified data lake. It supports online drafting, sharing, editing, and real-time referencing of documents. Users can easily create, version control, and approve documents in an intuitive collaborative manner within the organization and throughout the entire value network. This web-based writing environment brings users a rich interactive experience and truly enables collaborative development of technical documents.
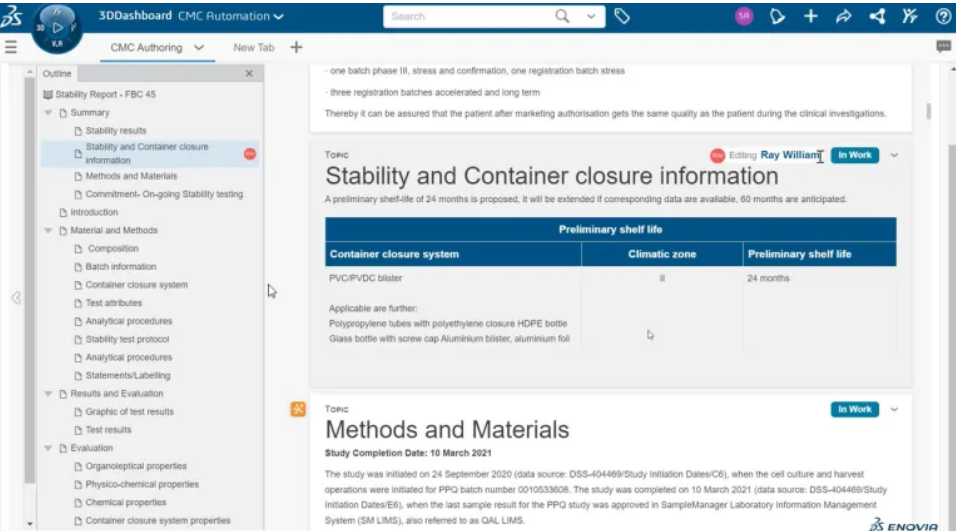
Diagram: Structured document view, where different users can independently edit, freeze, or publish different topics
Reference 1:https://www.fda.gov/media/131130/download
Reference 2:https://www.fda.gov/media/169611/download
Source: Dassault Systemes