System disconnection leads to errors and compliance risks

With the rapid development of the biopharmaceutical industry, it is crucial to maintain product quality and safety in the face of complex supply chains, strict regulatory requirements, and rapidly changing market demands. Regulatory agencies require that product approval should be based on strict quality management processes, emphasizing the predictability of the production process and high-frequency review mechanisms to increase output and reduce errors. As products mature, data analysis often becomes cumbersome and prone to errors.
Case sharing:
The transformation path of a biopharmaceutical company in Ireland
A certain Irish biopharmaceutical company experienced low work efficiency, increased compliance risks, and operational delays due to the isolation and disconnection of its systems, frequent human errors, and lack of visibility into global laboratories and production bases.
The company has faced many quality management challenges, such as:
1. The preparation of the annual quality report takes a long time and the audit process is inefficient
2. Repeated data entry leads to frequent errors and high difficulty in tracking events
3. Chemical test results are unpredictable
They urgently need a solution that can reliably collect data, report production activities, track various events, and have efficient data retrieval capabilities. They requested that the solution be integrated with their laboratory execution system BIOVIA ONE Lab automation to advance quality oriented investigation work.
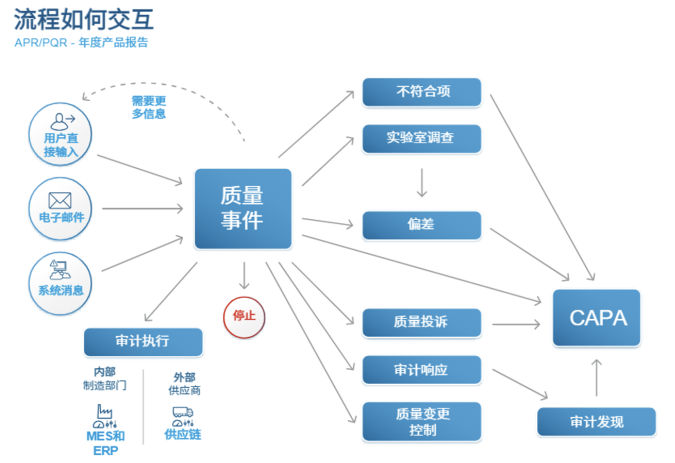
Figure: A series of associated process models for recording and testing in biopharmaceutical companies
Integrated quality management achieves significant efficiency improvement
BIOVIA Biopharma Quality Management Analyst helped the company successfully build a cross departmental integrated quality management system.
This solution utilizes pre-defined and validated process models and analysis tools to closely link corrective actions with root causes, achieving comprehensive automation of data collection, tracking, and reporting.
This solution helps the enterprise identify potential risks, improve inspection accuracy, enhance overall efficiency, and ensure adaptability in the constantly changing regulatory environment by minimizing human errors and providing a unified data view.
1. Improve productivity and eliminate human errors
2. Ensure consistent and interconnected processes to improve overall operational efficiency
3. Avoid duplicate data entry, reduce manual errors, and lower risks
4. Efficiently reuse collected data to meet diverse needs and improve data management efficiency
Scan the QR code to explore the complete case study:

Embracing virtual twin technology and promoting new changes in quality management
The use of virtual twin technology to improve efficiency and innovate with the rapid development of technology has fundamentally changed the way we handle critical processes, covering multiple dimensions such as production batches and quality control process development, drug discovery, and clinical trials. Virtual twin technology, as a digital mirror of key processes, enables precise simulation of real-world operations in the virtual world, in order to deeply explore potential optimization spaces. This simulation technology achieves in-depth analysis that traditional methods cannot compare to. It is not only a testing ground for new ideas, but also an early warning of potential problems, ensuring that we can avoid problems in real environments.
BIOVIA Biopharma Quality Management Analyst , A product based on 3DExperience ® The AI driven solution of the platform has tailored a unique set of features for biopharmaceutical companies, leading them from "passive problem solving" to "active problem avoidance".
It adopts model driven artificial intelligence, fully utilizes historical process intelligence, can accurately predict and effectively avoid potential problems, significantly improving the decision-making ability of the quality department. It relies on virtual twin technology to achieve quality control over the real world.
This solution is particularly suitable for biopharmaceutical companies that require interdisciplinary management of product and process quality. It is related to 3DExperience ® The vast business ecosystem on the platform seamlessly integrates, helping the company build a comprehensive and interconnected digital quality system, thereby achieving all-round visualization and deep insights.
Source: Dassault Systemes